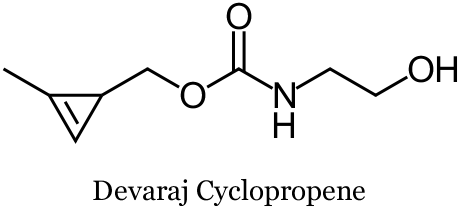
The relatively small size of lipids makes their properties very sensitive to chemical modifications. Recently, Devaraj and co-workers at the University of California, San Diego, developed a cyclopropene strained-dienophile in search of a tag with minimal steric impact to engage tetrazines in the SPIEDAC reaction. They found that the cyclopropene phospholipid could be imaged with a tetrazine probe in live human breast cancer cells, and obtained images similar to those obtained with a trans-cyclooctene (TCO) phospholipid.
Bioorthogonal chemical ligation reactions have been applied in the labeling of lipids. This includes the use of the copper-catalyzed azide-alkyne cycloaddition (CuAAC), the strain-promoted alkyne-azide cycloaddition (SPAAC), and the strain-promoted inverse electron-demand Diels-Alder cycloaddition (SPIEDAC). Bioorthogonal tags have been incorporated into lipid headgroups, as well as lipid acyl chains via synthetic methods and methods exploiting biosynthetic pathways. These studies have included phosphatidyl choline (PC), diacylglecerol (DAG), phosphatidic acid (PA), and phosphatidylinositol polyphosphate (PIPnS) lipids. These strategies have had success both in living cells and in vivo.
Of particular challenge in the design of tagged lipid analogs is the relatively small size of these molecules, making their properties very sensitive to chemical modifications. Recently, Devaraj and Co-workers at the University of California, San Diego, developed a cyclopropene strained-dienophile in search of a tag with minimal steric impact to engage tetrazines in the SPIEDAC
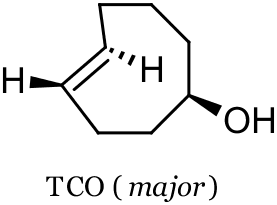
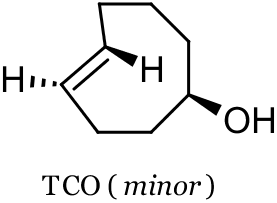
reaction. They found that the cyclopropene phospholipid could be imaged with a tetrazine probe in live human breast cancer cells, and obtained images similar to those obtained with a trans-cyclooctene (TCO, see below) phospholipid. Devaraj and co-workers found that the parent cyclopropene (see below) was stable in aqueous solutions containing L-cysteine at 60 oC, whereas the parent
TCO isomerized slowly to cis-cyclooctenol. Also highlighted is the utility of the fluorogenic tetrazine probe tetrazine-BODIPY FL, a probe with inherently low background fluorescence from nonspecific binding or accumulation. The tetrazine moeity’s ability to significantly quench fluorescence leads to fluorescent “turn-on” upon consumption of the tetrazine in the SPIEDAC reaction.
For more information about these reagents and techniques, please see the references below. For related topics please explore our collection of articles.
References:
- “Live-Cell Imaging of Cyclopropene Tags with Fluorogenic Tetrazine Cycloadditions” Yang, J.; Šečkutė, J.; Cole, C. M.; Devaraj, N. K. Angew. Chem. Int. Ed. 2012, 51, 7476-9.
- “Bioorthogonal Chemical Reporters for Analyzing Protein Lipidation and Lipid Trafficking” Hang, H. C.; Wilson, J. P.; Charron, G. Acc. Chem. Res. 2011, 44, 699-708.
- “Exploiting Bioorthogonal Chemistry to Elucidate Protein-Lipid Binding Interactions and Other Biological Roles of Phospholipids ” Best, M. D.; Rowland, M. M.; Bostic, H. E. Acc. Chem. Res. 2011, 44, 686-98.
- “Metabolic labeling and direct imaging of choline phospholids in vivo ” Jao, C. Y.; Roth, M.; Welti, R.; Salic, A. Proc. Natl. Acad. Sci. USA 2009, 106, 15332-7.
- “Selective Fluorescence Labeling of Lipids in Living Cells” Neef, A. B.; Schultz, C. Angew. Chem. Int. Ed. 2009, 48, 1498-500.