Inherently low concentration of biomolecules in living systems combined with limitations on achieving high concentration of labeling reagents due to toxicity, limited bioavailability, or further perturbations this introduces to the biological system, place great importance on a bioorthogonal reaction’s rate constant for its successful application in vivo.
Bioorthogonal reactions are distinguished by their ability to proceed in the context of biological systems. This requires that the reaction partners neither interact with nor interfere with the plethora of functional groups in the biological milieu on the required experimental time scale. For these reactions to be at all practical they need to also adhere to the criteria of “click” chemistry, whereby reactions are sufficiently selective, high yielding, and have good reaction kinetics. In addition, these reactions must typically proceed in water and at near-ambient temperatures (4-37oC). Several such reactions have been developed, most of which are employed in the ligation of biomolecules to chemical probes. In these strategies, a bioorthogonal reaction partner, referred to as a chemical reporter, is incorporated into a biomolecule chemically or biochemically. Ideally this modification results with minimal perturbation to the biophysical properties of the biomolecule, and provides the modified biomolecule in its native environment. The modified biomolecule is then treated with a probe molecule bearing the complementary bioorthogonal reaction partner to achieve conjugation. Since these reactions typically follow second-order kinetics, their rates depend on the concentrations of the reactive partners and the second-order rate constant of the reaction. The inherently low concentration of biomolecules in living systems combined with limitations on achieving high concentration of labeling reagents due to toxicity, limited bioavailability, or further perturbations this introduces to the biological system, place great importance on a bioorthogonal reaction’s rate constant for its successful application in vivo.
Several bioorthogonal reactions have been applied in vivo. These include the Staudinger ligation, the copper-catalyzed azide-alkyne cycloaddition (CuAAC), the strain-promoted alkyne-azide cycloaddition (SPAAC), and the strain-promoted inverse electron-demand Diels-Alder cycloaddition (SPIEDAC).
The Staudinger ligation of an azide and a triarylphosphine involves the strategic placement of an ester group on one of the phosphine’s aryl substituents, allowing an aza-ylide intermediate to undergo an intramolecular amide bond formation. This reaction has been found to proceed with a second order rate constant in the range of 0.00012 – 0.00024 M-1s-1.

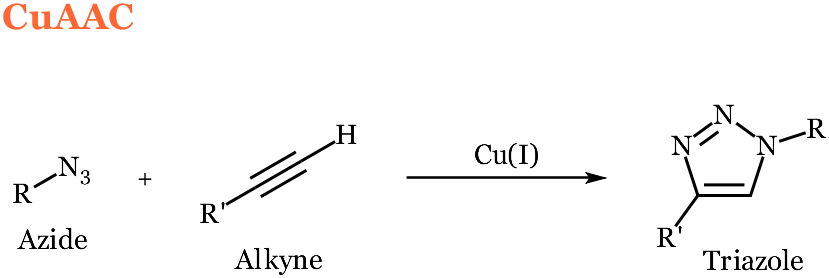
The copper-catalyzed azide-alkyne 1,3-dipolar cycloaddition (CuAAC) involves the union of a terminal alkyne and an azide to form a 1,4-disubstituted 1,2,3-triazole. Non-toxic Cu(I) catalyst-ligand combinations have been identified making this reaction applicable in living systems. These reactions have been found to proceed with a second order rate constant in the range of 0.0001 – 0.01 M-1s-1.
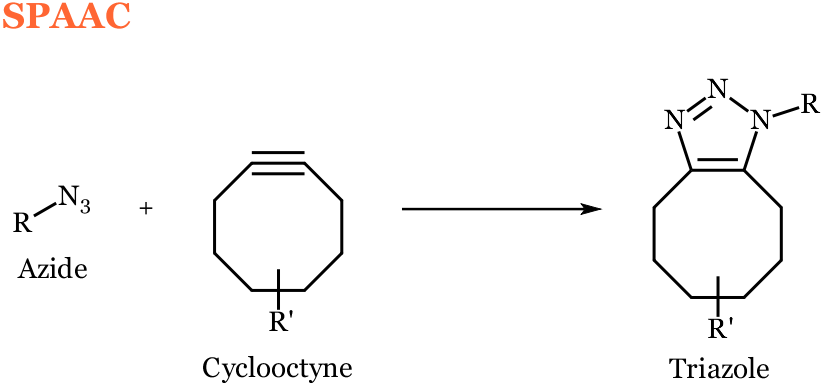
The strain-promoted alkyne-azide cycloaddition (SPAAC), also referred to as copper free click chemistry, involves the union of a strained alkyne, usually presented in a cyclooctyne structural motif, and an azide to form a triazole. These reactions have been found to proceed with a second order rate constant in the range of 0.0013 – 0.96 M-1s-1.
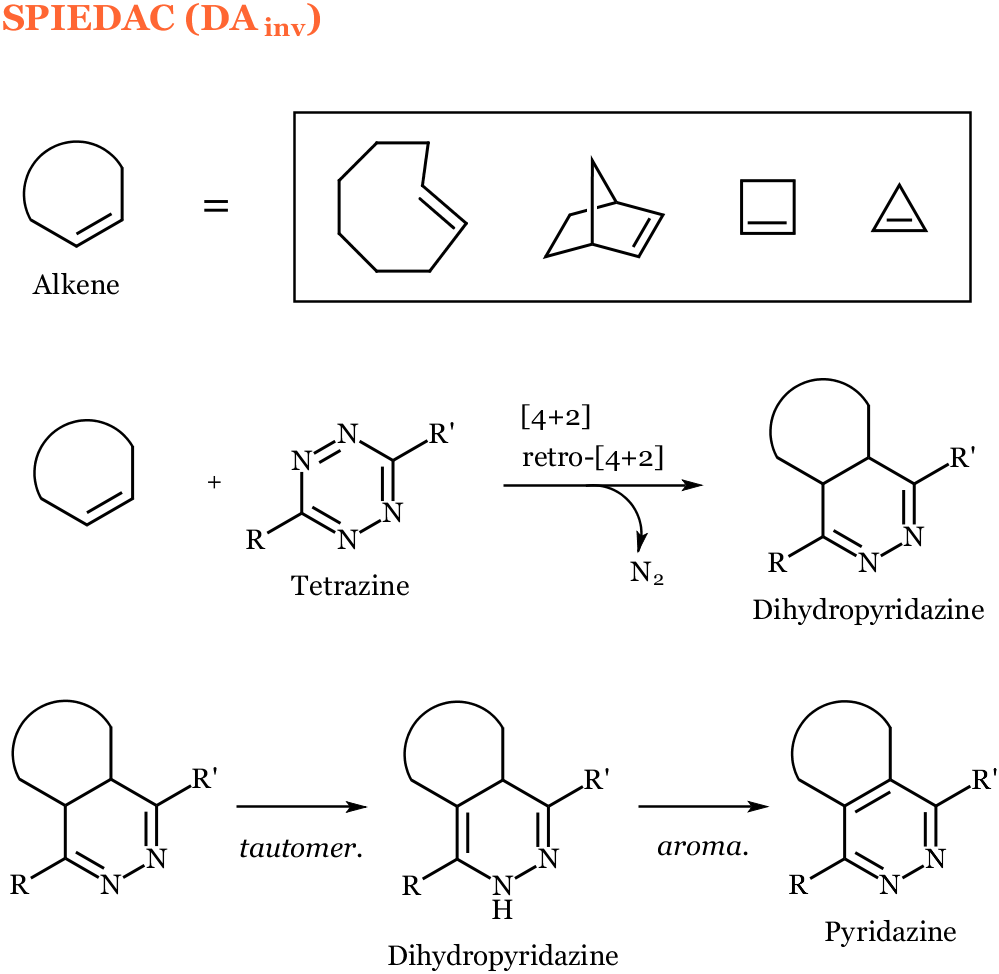
The strain-promoted inverse electron-demand Diels-Alder cycloaddition (SPIEDAC), also referred to as the inverse electron-demand Diels Alder cycloaddition (DAInv), involves the union of a 1,2,4,5-tetrazine and a strained alkene such as a trans-cyclooctene, norbornene, cyclobutene, or cyclopropene to form a cycloadduct that extrudes dinitrogen to give a dihydropyridazine. This dihydropyridazine has been reported to tautomerize yielding a mixture of isomers, as well as to aromatize to the corresponding pyridazine. DAInv reactions between trans-cyclooctenes (TCO) and tetrazines (Tz) have been found to proceed with a second order rate constant in the range of 210 – 30000 M-1s-1.
For more information about these reagents and techniques, please see the references below. For related topics please explore our collection of articles.
References:
- “A Need for Speed: Genetic Encoding of Rapid Cycloaddition Chemistries for Protein Labeling in Living Cells” Schmidt, M. J.; Summerer, D. ChemBioChem 2012, 13, 1553-7.
- “Bioorthogonal Reaction Pairs Enable Simultaneous, Selective, Multi-Target Imaging” Karver, M. R.; Weissleder, R.; Hilderbrand, S. A. Angew. Chem. Int. Ed. 2012, 51, 920-2.
- “Bioconjugation with Strained Alkenes and Alkynes ” Debets, M. F.; van Berkel, S. S.; Dommerholt, J.; Dirks, A. J.; Rutjes. F. P. J. T.; van Delft, F. L. Acc. Chem. Res. 2011, 44, 805-15.
- “Biomedical Applications of Tetrazine Cycloadditions” Devaraj, N. K.; Weissleder, R. Acc. Chem. Res. 2011, 44, 816-27.
- “From Mechanism to Mouse: A Tale of Two Bioorthogonal Reactions” Sletten, E. M.; Bertozzi, C. R. Acc. Chem. Res. 2011, 44, 666-76.
- “Synthesis and Evaluation of a Series of 1,2,4,5-Tetrazines for Bioorthogonal Conjugation” Karver, M. R.; Weissleder, R.; Hilderbrand, S. A. Bioconjugate Chem. 2011, 22, 2263-70.
- “High-Yielding, Two-Step 18F Labeling Strategy for 18F-PARP1 Inhibitors” Keliher, E. J.; Reiner, T.; Turetsky, A.; Hilderbrand, S. A.; Weissleder, R. ChemMedChem 2011, 6, 424-7.
- “Cu-free click cycloaddition reactions in chemical biology” Jewett, J. C.; Bertozzi, C. R. Chem. Soc. Rev. 2010, 39, 1272-9.
- “Tetrazine-trans-Cyclooctene Ligation for the Rapid Construction of 18F Labeled Probes ” Li, Z.; Cai, H.; Hassink, M.; Blackman, M. L.; Brown, R. C. D.; Conti, P. S.; Fox, J. M. Chem. Comm. 2010, 46, 8043-5.
- “Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality” Sletten, E. M.; Bertozzi, C. R. Angew. Chem. Int. Ed. 2009, 48, 6974-98.